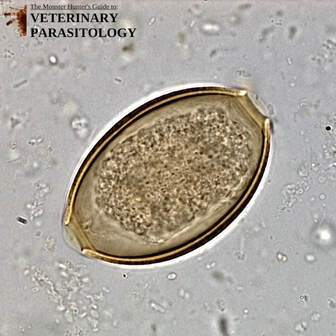
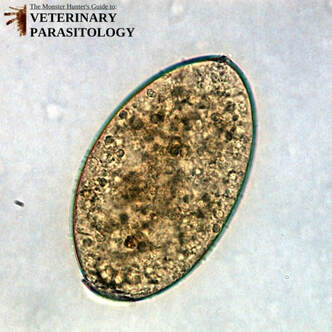
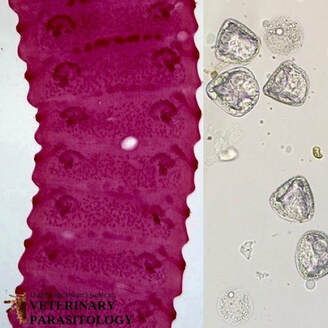
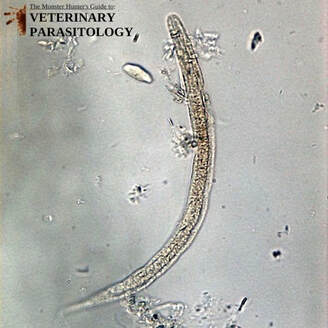
Dictyocaulus spp.(aka., Lungworm) |
Method of Detection:
Size:
|
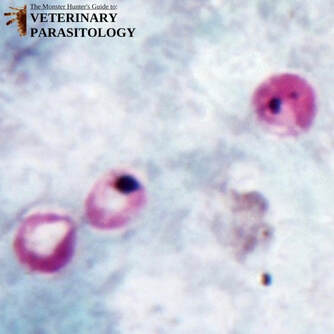
Eimeria cameli(aka., Coccidia) |
Method of Detection:
Size:
|
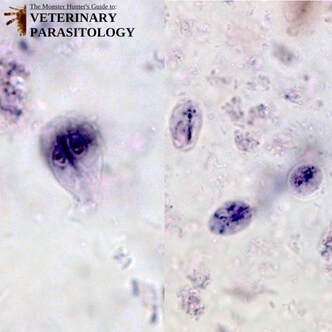
Eimeria macusaniensis(aka., E. mac, Big Mac, Coccidia) |
Method of Detection:
Size:
|
Fascioloides magna(aka., Large American Liver Fluke) |
Method of Detection:
Size:
|
Nematodirus spp. or Marshallagia spp.(aka., Thread-Necked Strongyle) |
Method of Detection:
Size:
|
Paramphistomum spp.(aka., Rumen Fluke) |
Method of Detection:
Size:
|
Skrjabinema spp.(aka., Pinworm) |
Method of Detection:
Size:
|
'Strongyle' Type Ova(aka., Haemonchus, Cooperia, Trichostrongylus, Teladorsagia, Oesophagostomum, Ostertagia, Bunostomum, Camelostrongylus, and Lamanema.) |
Method of Detection:
Size:
|
Trichuris spp.(aka., Whipworm) |
Method of Detection:
Size:
|
Sources Cited:
- Zajac, Anne M., and Gary A. Conboy. Veterinary Clinical Parasitology. 8th ed. West Sussex: John Wiley & Sons, 2012. Print.
- Taylor, Mike A., R. L. Coop, and Richard L. Wall. Veterinary Parasitology. 4th ed. Chichester, West Sussex: Wiley Blackwell, 2016. Print.
- American Association of Veterinary Parasitologists. (2010). Retrieved 2019, from https://www.aavp.org.
- Radfar, Mohammad H., and Mansour A. Gowhari. “Common gastrointestinal parasites of indigenous camels (Camelus dromedarius) with traditional husbandry management (free-ranging system) in central deserts of Iran.” Journal of Parasitic Diseases 37.2 (2013): 225–230. Web. 2 Feb. 2019.
- Cebra, Christopher K., and Bernadette V. Stang. “Comparison of methods to detect gastrointestinal parasites in llamas and alpacas.” Journal of the American Veterinary Medical Association 232.5 (2008): 733–741. Web. 2 Feb. 2019.